World
The wonder of whisky

In 2017, a guest at a luxury hotel in the Swiss resort of St Moritz bought the most expensive glass of whisky ever poured: a dram of an 1878 Macallan single malt that cost 9999 Swiss francs (£7600). Soon after, the hotel had given the guest a full refund. The whisky was a fake.
The signs had been picked up by experts who saw a photo of the bottle. The cork was wrong, the label looked too new, the certificate of authentication had a simple mistake obvious to a seasoned connoisseur. Testing showed it was a blended scotch, while carbon dating later confirmed a 95% probability the whisky had been created in the 1970s.
As the leading internationally traded spirit in the world, with Scotch worth £5.6 billion a year to the UK economy alone, it’s easy to see why whisky has attracted attention of counterfeiters. And the ‘Macallan’ is far from the only whisky fake to emerge. In 2018, the Scottish Universities Environmental Research Centre at the University of Glasgow, UK, found 21 supposedly rare Scotches – valued at £635,000 – were fake, with an estimated £41 million fakes in circulation. ‘The fascinating thing is that they’re not faking whisky,’ explains William Peveler, a chemistry lecturer at Glasgow. ‘If you don’t know what whisky tastes like, you’re just out to get some fancy-looking brown liquid in a fancy glass bottle, then a bit of caramel and some water will do you right. As long as it’s alcoholic, pungent and brown, there’s a certain amount of buyers who’d never know they’d been had.’
Faking alcohol is nothing new. In Ancient Rome, Pliny the Elder complained about the number of fake wines flooding the market, noting that Falernian wine could be identified as ‘it is the only wine that takes light when a flame is applied to it’ due to its high alcohol content. Today, wine fraud amounts to hundreds of millions in losses, thanks to the relatively easy nature of wine to fake: colouring, flavouring and blending are simple. The problem is so vast that in 2007 the Italian police even trained 25 officers as sommeliers to help spot bad booze.

Whisky has its own safety challenges – most notably in India, the world’s biggest market for the drink, which accounts for around half of the world’s consumption, some 1.5 billion litres a year. This has led to illicit manufacture of whisky to meet demand, particularly in Bihar, Gujurat, Mizoram and Nagaland, states where alcohol is prohibited. While most whisky adulterants are relatively harmless – water, desi daru (a type of Indian liquor) or cheaper brands – hundreds have died from contaminants.
This risk to life, and wallets, means whisky experts have had to develop detailed analytical techniques to fight fraud. They’re also starting to unlock the mysteries of a drink that, although made from simple ingredients – just cereal, water, yeast and caramel colouring – is arguably the most chemically complex tipple in the world.
Complex notes
Mimicking the taste of whisky is far more complicated than simple adulteration. ‘It’s typically defined by custom and law, rather than chemistry,’ says Peveler. ‘Ultimately, what you’re making is a distilled spirit – effectively a vodka: ethanol and water. The things that you are in control of is what you make that spirit out of, and the residual chemistry. It’s not a perfect distillation process, and that’s by design.’
‘There are three key areas for flavour development,’ explains Ian Goodall, a senior scientist at the Scotch Whisky Research Institute (SWRI), UK. ‘Fermentation, the action of the yeast forming alcohol from the sugars. So you’ve got esters, aldehydes, acids, ketones and disulfides.’
Next, distillation starts the chemical reaction through heat and the action of copper stills, and produces three different fractions. The foreshot, with volatile acetaldehyde and ethyl acetate, is removed and the feint, which includes low-volatility compounds such as phenols and nitrogen compounds, is redistilled. The middle, or spirit, fraction (different distilleries start and end collection of spirit at different points) then moves on to the final step: maturation.
It’s here that chemical complexity truly develops. By law, Scotch whisky has to be matured for at least three years. Thanks to Scotland’s damp and colder climate, this reduces the concentration of ethanol inside the cask; bourbons in the US, by contrast, increase their ethanol concentration. ‘Maturation isn’t just a single process, or one overriding influence,’ says John Conner, the SWRI’s lead on maturation research. ‘First, you’ve got what you put in the cask. But the type of cask you put it in has a big influence as well. And, if you’re keeping whisky for a length of time, the reactions do not stay constant: as you age longer, the proportion of your extractives tends to change.’
Whisky casks have a capacity of less than 700 litres, as maturation requires good contact with the wood. Oxygen diffuses into the cask’s wood (we still aren’t sure precisely how this occurs), which then triggers a whole host of new reactions – most notably the formation of esters. Perhaps most notably, cellulose from the casks is broken down, releasing hemicellulose and lignin, which give the spirit its distinctive brown colour. Scotch has another twist, too, Conner explains. ‘We don’t use new casks. We traditionally refill ones that have been used by the sherry and bourbon industries. After that first use, it reduces the amount of extract you get from the cask. That’s why transformation reactions become a bit more important. It’s that slow build-up of time, coupled with the lower intensity that you get from wood [that gives Scotch its flavour].’
All of this is before the master blender decides how many casks to use to make the final blend of substances, or congeners, in the whisky – different molecules, different notes, different concentrations. And it’s precisely this reason that makes whisky almost impossible to fake at a chemical level, explains Peveler. ‘Chromatography will look different – any fingerprint you try and derive. That kind of pattern matching for fraud is very tricky, because you’d have to have an enormous database.’
Whisky in the NMR
This hasn’t stopped the counterfeiters from trying, so the industry has fallen back on reliable methods of detection: gas chromatography, mass spectrometry, HPLC or, for low concentrations of analytes, solid-phase microextraction (SPME). These can struggle to give an accurate picture of components, although chemical analysis is moving forward. In 2018, a team led by Kenneth Suslick at the University of Illinois at Urbana–Champaign, US, developed an ‘optoelectronic nose’ for identifying liquors. Expanding on work previously used to identify explosives, Suslick devised an array of 36 cross-reactive inks that detects molecules such as aldehydes and ketones, as well as indicating the sample’s pH. Samples are partially oxidised before exposure to the array, which results in more chemically reactive molecules such as quinones and carboxylic acids, then they flow through the array, reacting with the inks to create a unique colour profile. This can then be matched to the existing database to confirm whether the whisky is genuine.
These tests only go so far, however. While authentication is important, they don’t tell us how these molecules are formed, or the processes underway as the whisky is aged. It’s understanding these chemical processes that will allow us to unlock whisky’s mysteries.
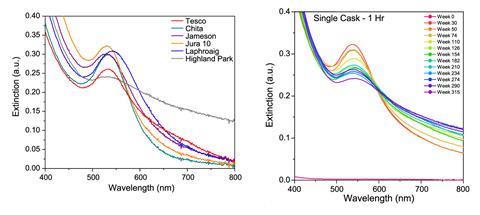
Goodall has been working on this issue. In 2020, in collaboration with the University of Edinburgh, UK, he used 1H NMR spectroscopy to analyse whisky’s congeners. Previously, the complexity of whisky’s spectra, and the sensitivity of NMR, made it difficult to analyse. But, with small samples of only 500µl, and by supressing the signals for water and ethanol that would otherwise make the spectra hard to distinguish, the team were able to determine the exact concentration of 13 out of 21 congeners.
This isn’t the only solution being explored. ‘I love a HPLC, but they’re big, expensive and sit on a bench somewhere, where you have to send samples away,’ says Peveler. ‘I’ve been looking at in situ sensing solutions, and you don’t need to know about every molecule in a sample to know what’s going on.’
Peveler’s approach is to take a sample from the cask, then mix it with an aqueous gold(iii) solution to form gold nanoparticles. This results in a clear colour change thanks to surface plasmon resonance – the excitation of electrons in the gold from light. ‘It’s really distinctive, a colourless to red-to-purple colour change, depending on the size of the colloid and the local environment,’ Peveler says. And, as the different molecules form different sized and shaped nanoparticles, the nature of the colour change varies depending not just on the brand. ‘We raided our cupboards, and we got a whole bunch of whiskys from around the world – Scotland, American, Irish, even an English one. And they all produced different colours.’
We’ve tried spiking whisky. We’ve tried making fake whisky – and nothing really happened
Peveler’s test goes further. It produces results within 15 minutes, and he has found a clear link with age. ‘It’s not a case of going “Oh, this whisky has been in a barrel for three years”, it’s a case of how much chemistry has been happening in that barrel. We were very kindly given this wonderful sample set, where one cask was dipped every six months. And we could test those samples and see a really clear trend in how the chemistry changed as the whisky got older: the gold nanoparticles change drastically.’
This brings us back to the original question. If there are chemical indicators for ageing, surely they can be faked?
‘In principle,’ Peveler says. ‘But what’s interesting is that there is a complex chemical network. We’ve tried spiking whisky. We’ve tried making fake whisky, where we took a 40% alcohol solution and added some of the compounds that we thought would result in gold nanoparticle formation. And nothing really happened – at least nothing like we saw for [the real thing]. We’re missing something. There are a lot of clear candidates, but what’s driving this particular colour change, we haven’t got to, yet!’
Age-old problems
As ageing is a chemical process, it makes sense that it could, in theory, be sped up. Unsurprisingly, with whisky in high demand and the cost of space to store barrels prohibitive for whisky start-ups, these alternatives are already being explored. They usually fall into a handful of categories: either a high-pressure treatment, which is designed to push the alcohol through the barrel, causing the reactions necessary to pick up distinctive notes; injecting atmospheric oxygen into the whisky for improved exposure; or the use of ultrasound to disturb the liquid, create cavitation bubbles and cause oxygen-based reactions. ‘There is a lot of work going on now looking at innovation,’ says Goodall. ‘People are trying to improve the efficiency of the process. But Scotch and whisky are fairly well defined in European and UK legislation, which is there to essentially protect the quality of the product and ensure consumers know what they’re getting.’
It’s very hard for that chemistry to exist without ageing in Scotland
That these ideas aren’t taking the whisky market by storm isn’t just down to legal protection. In 2022, a team at the University of Northern Colorado in the US looked at these accelerating ageing processes using GC–MS, testing barrel-aged whiskies and those created with accelerated ageing techniques to determine the compounds present. The result was entirely different chemical reactions had occurred. While barrel-aged whisky is an additive process, ‘accelerated ageing processes also appear additive but result in differing or additional compounds’. While the treatments (except atmospheric injections) resulted in chemical reactions, the researchers concluded it wasn’t mimicking ageing. ‘If you look at things like ultrasound,’ Conner says, ‘it’s basically just trying to get the extract out of the wood quicker. There are some that are slightly more sophisticated and have tried to take out the immature character [of the whisky] as well, but you can’t replicate [the ageing process].’ Peveler agrees. ‘If you’re expecting a certain concentration of compounds from a whisky,’ he says, ‘it’s very hard for that chemistry to exist without ageing in Scotland for a minimum of three years.’
But that statement also indicates the real threat to the future of whisky’s chemical complexity: climate change. If you change the location of the barrel, the environment alpso changes. One of the reasons that India hasn’t been able to create a home-grown industry with the same flavour profiles is that the climate – hot and dry, which results in water evaporating before alcohol – doesn’t produce the same distinctive notes as a damp and cool Scotland. This, along with changing crop yields for ingredients, water shortages and as-yet unidentified challenges mean the whisky industry is already starting to futureproof itself against climate shock. ‘Sustainability is our biggest challenge at the moment,’ Goodall says. ‘We’re looking at our environmental credentials and decarbonising the process.’
Right now, though, that is a problem that even an industry built on thinking years ahead can’t tackle alone. And the challenge of authentication – and unlocking the chemical fingerprints of whisky – is never far from a distiller’s mind. ‘But I would like a machine that would be able to detect a non-authentic product through the bottle,’ Goodall adds. ‘It would simplify things considerably.’
Kit Chapman is a science journalist based in Falmouth, UK